
PRECISE II was a nonrandomized, blinded, prospective, single-arm, multicenter study that evaluated the accuracy and safety of the implantable Eversense CGM system among adult participants with T1D and T2D. Entry Author and DateAllison Hare, April 2021 Glucose data syncs with monitor to provide graph-based visualization of continuous glucose levels, and can send data to up to 10 people including clinical team members. Customizable alerts when glucose levels fall out of range
#BIOTEL HEART MONITOR RASH SKIN#
Quality ControlĪ sensor continuously measures glucose levels just beneath the skin and sends data wirelessly to a display device through a transmitter. Performance of a Factory-Calibrated Real-Time Continuous Glucose Monitoring System Utilizing an Automated Sensor Applicator. Accuracy was maintained across 10 days of use and various glucose concentration ranges in both adults and children and adolescents. Reprocessed data from 62 participants (25 adults and 37 children and adolescents of ages 6–17 years 3532 YSI–CGM pairs) were analyzed.

Accuracy evaluation included the proportion of CGM values that were within ☒0% of YSI reference value for glucose levels >100 mg/dL and ☒0 mg/dL for YSI glucose levels ≤100 mg/dL (%20/20), the analogous %15/15 and %30/30, and the mean absolute relative difference (MARD) between temporally matched CGM and YSI values. In-clinic visits for frequent comparative blood glucose measurements using a reference instrument (YSI) were conducted on days 1, 4–5, 7, and/or 10 of system use.
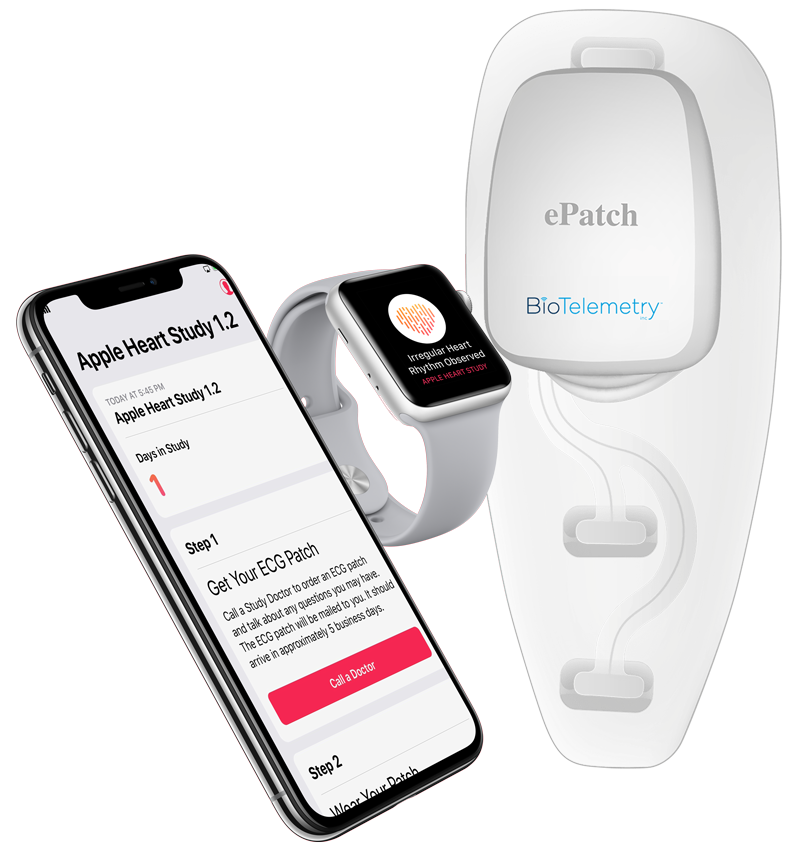
sites as part of a larger study of G6 system performance. Entry Author and DateAllison Hare, April 2021ħ6 participants with insulin-treated diabetes were enrolled at four U.S. Available for purchase from Amazon, CVS, Kroger, Rite Aid, Target, Walgreens, and Walmart. Digital glucose meter which syncs data to patient-facing app and has traffic light-style light system to easily convey to user whether their glucose level is within, nearing the limits of, or out of range.Journal of Diabetes Science and Technology. A New, Wireless-enabled Blood Glucose Monitoring System That Links to a Smart Mobile Device: Accuracy and User Performance Evaluation. Questionnaire results indicated that most subjects considered the system easy to use. Moreover, 97.6% (321/329) of subject self-test results were within ☑0 mg/dl (☐.6 mmol/L) or ☑0% of the YSI reference result.
#BIOTEL HEART MONITOR RASH ISO#
In the clinical study, among subjects with diabetes, 99.4% (327/329) of subject self-test results, 99.7% (331/332) of results obtained by study staff, 97.2% (309/318) of subject palm results, and 100% (330/330) of venous results met ISO 15197:2013 Section 8 accuracy criteria. In the laboratory study, 100% (600/600) of combined results for all 3 test strip lots met ISO 15197:2013 Section 6.3 accuracy criteria. Meters can be purchased from vendor site.
#BIOTEL HEART MONITOR RASH FREE#
Technical Aspects/Integrationĭevices sync to mySugr app via Bluetooth, which is free with purchase of meters. "Guide" model has a back-lit display, unlike "Guide Me" model. The Accu-Chek Mobile blood glucose monitoring system used under controlled conditions meets ISO 15197 standards in the hands of diabetes patients. Sachse D, Bolstad N, Jonsson M, Sæves I, Johansson CB, Delezuch W, Hagve M, Hardang IM, Isaksson HS, Ivarsson A, Lehto L, Keikkala E, Mattsson N, Ranta JK, Stavelin A, Sudmann AA, Varsi K. Of the glucose concentrations obtained by both the scientist and the patients, more than 95% of the individual results were within ± 20% of the comparative method, meeting the ISO 15197 accuracy goal, but not the stricter ± 10% ADA goal. The meter yields reproducible readings, with an imprecision CV <5% as required by the American Diabetes Association (ADA). The patients answered a questionnaire about the ease of use of the meter. Patient samples, containing glucose in concentrations mainly between ˜4 and ˜20 mmol/L, were analyzed in duplicates both on the Accu-Chek Mobile and with the comparative method. Diabetics (N = 88) with previous experience of self-testing were recruited for the study.

The designated comparative method was a hexokinase-based laboratory method (Architect ci8200). The performance of the Accu-Chek Mobile was evaluated both in the hands of a scientist and of diabetes patients.
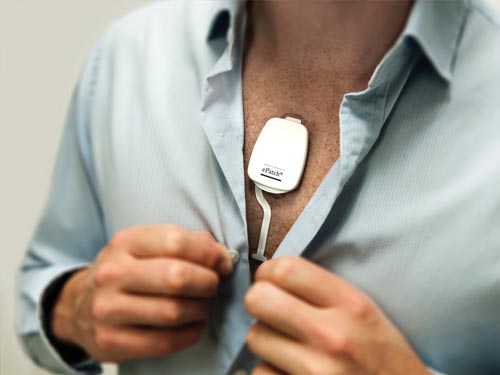
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.


 0 kommentar(er)
0 kommentar(er)
